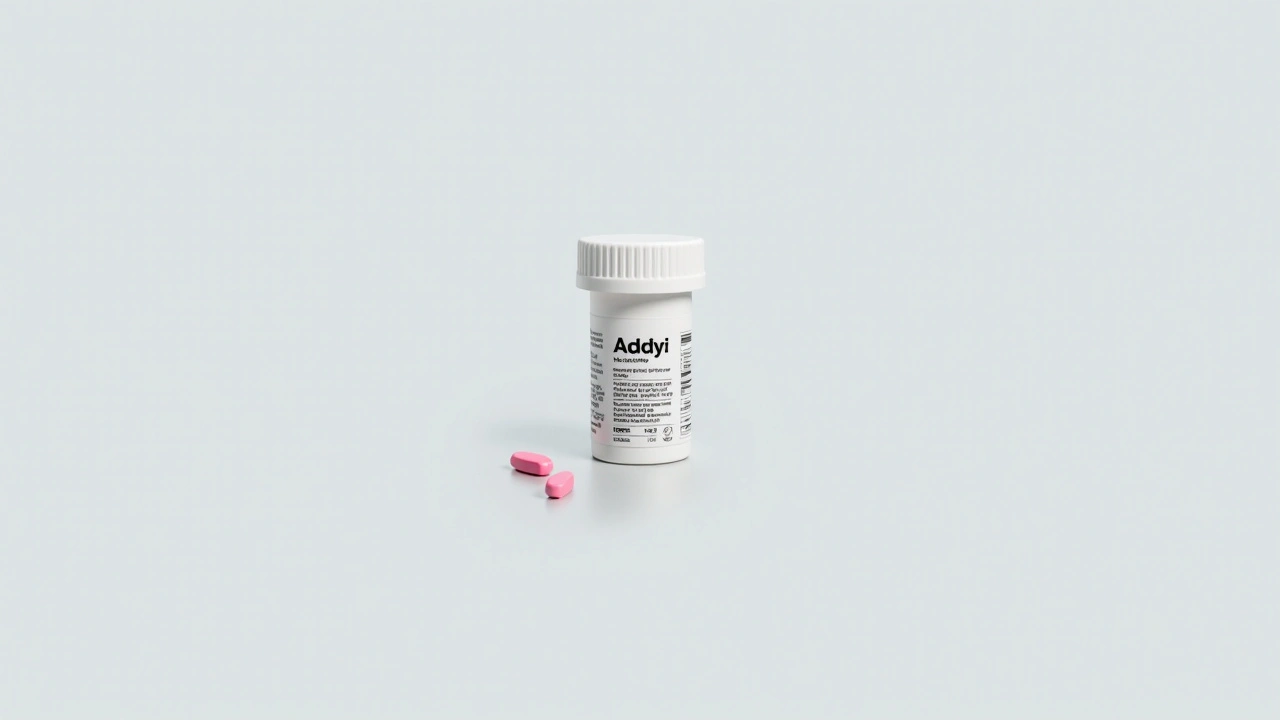
FDA Approves Controversial 'Little Pink Pill' Amid Various Restrictions
The Food and Drug Administration (FDA) recently gave its nod to flibanserin, widely dubbed the 'little pink pill,' a medication aimed explicitly at increasing the sexual desire in women. Sold under the brand name Addyi, this drug has been designed to address Hypoactive Sexual Desire Disorder (HSDD). This approval comes after a lengthy and intense lobbying effort by the drug's producers and certain advocacy groups.
A Detailed Look at Hypoactive Sexual Desire Disorder (HSDD)
HSDD is a condition that impacts a significant number of women. Characterized by an ongoing lack of sexual interest, it leads to considerable distress or interpersonal difficulties. The key factor distinguishing HSDD is that this reduced desire is not attributable to any existing medical or psychiatric issues. Previously, treatment options were limited, with most focusing on hormonal solutions or couples therapy.
How Addyi Works Differently from Other Treatments
Unlike Viagra, which primarily works by increasing blood flow, Addyi operates on a different mechanism. Flibanserin modifies serotonin levels in the brain, a neurotransmitter known to affect mood and arousal. Early studies have indicated that the drug can increase the number of satisfying sexual events by approximately half to one per month over a placebo.
Expert Opinions: A Mixed Bag
Dr. Lauren Streicher from Northwestern University has lauded the approval, seeing it as a crucial step forward in providing non-hormonal therapeutic options for women plagued by distressingly low sexual desire. According to her, this marks a significant leap for women's health, offering new avenues for treatment that were previously unavailable.
However, not all experts share this enthusiasm. Dr. Philip Hanno, a urologist from the University of Pennsylvania, was among those who voted against recommending the drug for approval. Dr. Hanno and other critics have concerns about the efficacy and safety of the medication. Among the noted side effects are low blood pressure, dizziness, and sleepiness, which pose substantial risks to users.
The Influence of the Lobbying Campaign
There’s no denying that a robust lobbying campaign influenced the FDA's decision. Supported by the pharmaceutical company, the group 'Even the Score' played a significant role in bringing attention to the need for treatments addressing female sexual dysfunction. Critics argue that this intense lobbying may have swayed the FDA, leading them to prioritize approval despite lingering efficacy and safety concerns.
Stringent Restrictions: What Do They Entail?
One of the most notable aspects of the FDA's approval is the number of restrictions and conditions imposed on Addyi's prescription and use. Unlike other medications, Addyi will require healthcare professionals to go through special training and certification to prescribe it. Additionally, it will only be available through specific pharmacies, ensuring thorough monitoring and restricted use.
Market Availability and Pricing
The drug is slated to be available to the public in October. Pricing is expected to be on par with Viagra, making it relatively expensive. This move raises questions about accessibility for women from different socioeconomic backgrounds who may benefit from the medication but might find the cost prohibitive.
Weighing the Pros and Cons
While the approval of Addyi marks a significant milestone, it brings along a myriad of questions and concerns. On the one hand, it provides a new option for treating HSDD, a condition that has been inadequately addressed for years. On the other, issues related to its side effects, overall effectiveness, and the ethics behind its approval process cannot be ignored.
The debate over Addyi is far from over. As the drug reaches the market and begins to be used by the public, further data will likely provide a clearer picture of its true benefits and complications. Until then, discussions around its approval will remain charged and multi-faceted, marking an intersection of science, ethics, and advocacy.



Wendy Tharp
August 21, 2024 at 02:54
This is just another example of Big Pharma selling us a pill for a problem they invented. Women aren't broken because they don't want sex every damn day. Stop medicalizing normal life.
And don't even get me started on the 'Even the Score' campaign. It's not equality if you're just giving women a chemical crutch to pretend they're as horny as men.