Have you ever picked up a prescription and noticed the pill looks different - maybe a different color or shape - but the name on the bottle is the same? You might think it’s a new generic version. But what if it’s not a generic at all? What if it’s the exact same pill your doctor prescribed, just sold under a different label? That’s an authorized generic.
What Exactly Is an Authorized Generic?
An authorized generic is not a copy. It’s not a knockoff. It’s the real thing - made by the same company that makes the brand-name drug, in the same factory, with the same ingredients, same process, same quality control. The only difference? The label doesn’t have the brand name on it.
Think of it like this: You buy a Coca-Cola from the store. Now imagine Coca-Cola also sells the exact same soda in a plain bottle with no logo, just labeled as "cola." It’s not a different recipe. It’s not cheaper because it’s lower quality. It’s the same drink, just packaged differently to compete in the generic aisle.
The FDA defines an authorized generic as a drug that’s approved under the original brand’s New Drug Application (NDA), not a separate generic application. That means it skips the usual generic approval process. No bioequivalence studies. No waiting. It’s the brand drug, repackaged under a generic name.
How Is It Different From Regular Generics?
Regular generics are made by other companies after the brand’s patent expires. They must prove they work the same way as the brand - same active ingredient, same dose, same effect. But they can use different inactive ingredients - fillers, dyes, coatings - which sometimes changes the pill’s color, shape, or taste.
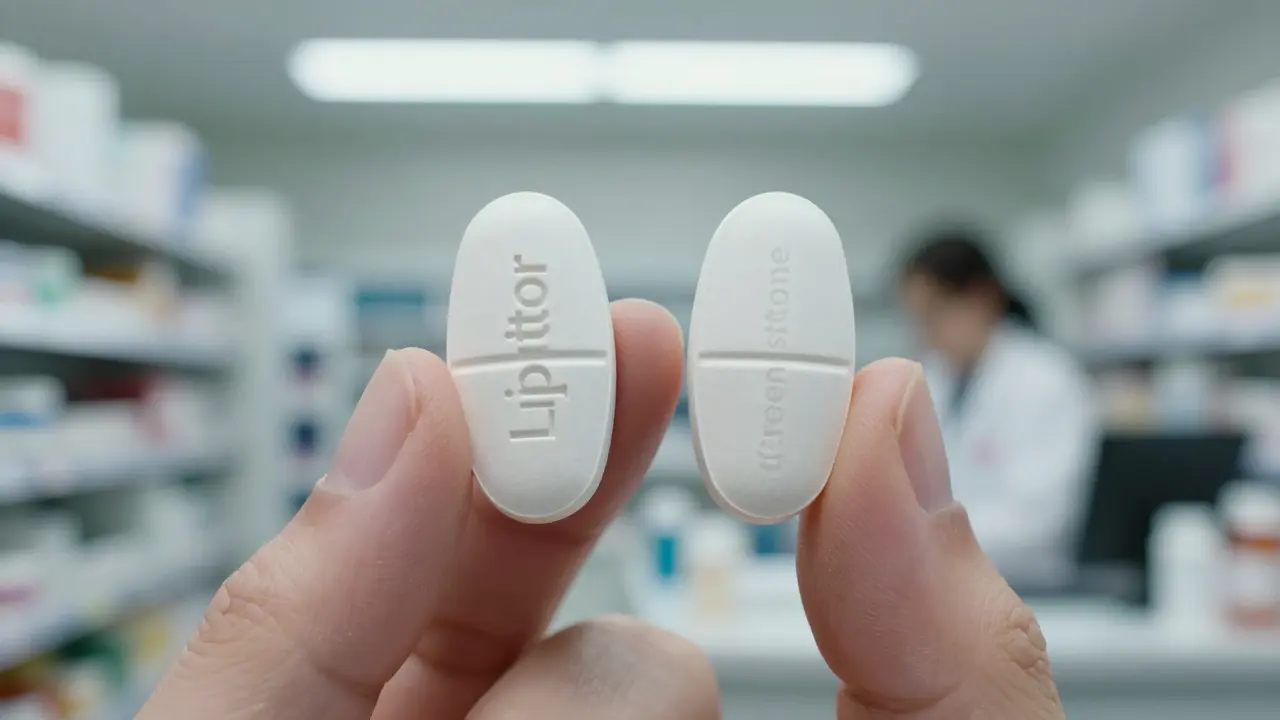
Authorized generics don’t have that flexibility. They must match the brand drug down to the last milligram of inactive ingredient. No substitutions. No changes. If your brand pill is white and oval with a "Pfizer" imprint, the authorized generic will be white and oval with a "Pfizer" imprint - unless the label says "Greenstone" instead. That’s it.
Here’s the catch: regular generics are listed in the FDA’s Orange Book. Authorized generics? Not there. That’s because they’re not approved as generics. They’re approved as the brand drug, just sold differently. So pharmacists can’t just look up "simvastatin" and see that Greenstone’s version is interchangeable. They have to know it’s an authorized generic - or check the FDA’s separate list.
Why Do Companies Make Authorized Generics?
It’s a smart business move - and it’s been around since the early 2000s. When a brand-name drug’s patent runs out, the first generic company to file gets 180 days of exclusive sales. That’s a huge financial reward. But the brand company doesn’t want to lose all that market share.
So they launch their own authorized generic - often right before or during that 180-day window. Now they’re selling the same drug at generic prices, but they’re still the ones making the profit. The first generic company? They’re suddenly competing with the original maker. That can crush their profits, and sometimes even scare off other generic companies from entering the market.
Companies like Pfizer (through Greenstone), Procter & Gamble (through Prasco), and others have built entire divisions just to make and sell authorized generics. They’re not trying to be cheap. They’re trying to stay in control.

What Does This Mean for You as a Patient?
Here’s the good news: you’re getting the exact same medication. No difference in how it works. No difference in side effects. No difference in safety. If your brand drug worked for you, the authorized generic will work the same way.
The bad news? You might not know you’re getting it. Pharmacists sometimes switch prescriptions automatically unless you say "no substitutions." And if the pill looks different, you might think something’s wrong. You might call your doctor. You might stop taking it. That’s not necessary - but it happens.
Some patients report confusion when they get a pill that looks nothing like the one they’re used to. Even if the name on the bottle is the same, the color or imprint changes. That’s normal with authorized generics. It’s not a mistake. It’s just the label.
And here’s something most people don’t realize: you might be paying less for the same drug. Authorized generics are often priced just like regular generics - sometimes even cheaper. But because they’re made by the brand company, they’re not always listed in the same discount programs. Check your pharmacy’s price list. Ask if they carry the authorized version. You might save money without sacrificing quality.
Why Aren’t Authorized Generics Listed in the Orange Book?
The Orange Book is the FDA’s official list of approved drugs and their therapeutic equivalents. It’s what pharmacists and doctors rely on to know which generics can be swapped for brand drugs.
But authorized generics don’t go through the Abbreviated New Drug Application (ANDA) process. They’re approved under the brand’s NDA. So they don’t get listed. That creates a real problem for pharmacists. They can’t just pull up a generic substitution report and see that "Simvastatin 20mg, Greenstone" is interchangeable. They have to know it’s an authorized generic - or look it up on the FDA’s separate Authorized Generic List.
This gap in the system means some pharmacists don’t even know they’re dispensing an authorized generic. And if they don’t know, they can’t tell you. So you might get one without realizing it. That’s not their fault. It’s a flaw in how the system is built.
Should You Ask for an Authorized Generic?
Yes - if you care about consistency and cost.
If you’ve had side effects with a regular generic - maybe it made you feel nauseous or gave you headaches - and you think it’s because of the inactive ingredients, an authorized generic might help. Since it’s identical to the brand, it’s less likely to cause those issues.
If you’re paying out of pocket and want the cheapest option, ask your pharmacy if they carry the authorized version. Sometimes it’s priced lower than the regular generic. Sometimes it’s the same price. But you’re getting the same drug as the brand - just without the logo.
And if you’re on a steady medication - like blood pressure pills, antidepressants, or thyroid meds - you want consistency. You don’t want your pill to change color every time you refill. An authorized generic gives you that stability.

What’s the Big Picture?
Authorized generics are a product of the Hatch-Waxman Act of 1984 - the law that created the modern generic drug system. It was meant to lower drug prices by encouraging competition. But it also created a loophole: brand companies can use the system to stay in the game after their patent expires.
Some experts argue this undermines the whole point of generics. If the brand company can just launch its own version and crush the first generic applicant, why would any company risk the cost and time of challenging a patent? That could mean fewer generics overall - and higher prices in the long run.
Others say it’s just good business. If you can offer the same drug at a lower price, why shouldn’t you? Patients benefit. Pharmacies benefit. Even insurers benefit.
The FDA doesn’t take sides. They just track it. They maintain a public list of authorized generics. They don’t regulate them differently. They treat them as the same as the brand drug - because, legally and chemically, they are.
How to Spot an Authorized Generic
You won’t always know. But here’s how to find out:
- Check the label. Look for the manufacturer name. If it’s Pfizer, Novartis, or another big brand, and the drug name matches your brand prescription, it might be an authorized generic.
- Ask your pharmacist: "Is this an authorized generic?" They can check the FDA’s list or their internal database.
- Use GoodRx or other price apps. Sometimes they’ll show "Authorized Generic" next to the price.
- Compare the pill imprint. If it’s identical to your brand pill - same color, same marking - it’s likely an authorized generic.
Don’t assume it’s a mistake. Don’t assume it’s inferior. Just ask. Knowledge is your best tool.
Final Thought: It’s Not About the Label. It’s About the Medicine.
At the end of the day, the pill in your hand doesn’t care what’s printed on the label. It doesn’t care if it’s called "Lipitor" or "atorvastatin" or "Greenstone atorvastatin." It works the same way. It’s absorbed the same way. It affects your body the same way.
Authorized generics aren’t a trick. They’re not a scam. They’re a reflection of how complex - and sometimes contradictory - the drug market has become. The system was built to save money. But sometimes, the people who built the system find ways to keep making money too.
What matters is this: you’re getting the same drug. You’re paying less. And you’re not sacrificing safety or effectiveness. That’s worth knowing. That’s worth asking for. And that’s worth talking about - not just with your pharmacist, but with your doctor, your insurer, and anyone else who helps you manage your health.
Are authorized generics as safe as brand-name drugs?
Yes. Authorized generics are made by the same manufacturer, in the same facility, with the same ingredients and quality controls as the brand-name drug. The FDA considers them therapeutically identical. The only difference is the label.
Can I switch from a brand to an authorized generic without side effects?
Yes. Because authorized generics are chemically identical to the brand drug, switching should not cause any new side effects. If you’ve had reactions to regular generics (like nausea or dizziness), an authorized generic may be a better option since it avoids differences in inactive ingredients.
Why does my pill look different if it’s the same drug?
Authorized generics can have different colors or markings to distinguish them from the brand-name version - but only because of labeling rules. The active ingredient, dosage, and all inactive ingredients remain unchanged. It’s the same medicine, just packaged differently.
Are authorized generics cheaper than regular generics?
Often, yes. Authorized generics are typically priced at or below the cost of regular generics because they’re made by the brand company and don’t require separate FDA approval. Sometimes they’re even the lowest-cost option available.
How do I know if my prescription was filled with an authorized generic?
Check the manufacturer name on the bottle. If it’s a known brand company like Pfizer (Greenstone), Procter & Gamble (Prasco), or another major pharmaceutical firm, it’s likely an authorized generic. Ask your pharmacist - they can check the FDA’s authorized generic list or their internal records.
If you’re on a long-term medication and want consistency, ask about authorized generics. If you’re trying to save money, ask if they’re available. You’re not asking for a downgrade - you’re asking for the same drug, without the brand markup.




Angela Stanton
January 9, 2026 at 08:04
Okay so let me get this straight - authorized generics are just brand drugs in a tuxedo without the logo? 🤯 FDA doesn't list them in the Orange Book? That’s like selling the same Tesla but calling it a ‘Model S Generic’ and hiding the logo under the hood. Pharma companies are playing 4D chess while we’re stuck in checkers. And yes, I’ve gotten Greenstone atorvastatin - looked different, panicked, called my doctor. Turned out it was *better*. Same pill, 40% cheaper. 📉💊