Drug Safety Comparison Tool
Compare 2024-2025 FDA Approvals
Select up to 3 drugs from the article to compare their safety profiles. This tool uses data from clinical trials and real-world monitoring.
Safety Comparison Results
| Safety Metric | Drug 1 | Drug 2 | Drug 3 |
|---|---|---|---|
| Serious Side Effects | |||
| Common Side Effects | |||
| Monitoring Required | |||
| Real-World Risk Factor | |||
| Overall Risk Level |
New Drug Approvals 2024-2025: What’s Approved and How Safe Are They?
In 2024, the FDA approved 50 new molecular entities-the highest number since 2018. That’s not just a number. It’s 50 new chances for people with serious, often untreatable conditions to get better. But with every breakthrough comes a question: How safe are these drugs really? The answer isn’t simple. Some are game-changers with clean safety records. Others come with warnings that require careful monitoring. This isn’t science fiction. These drugs are already in use. And more are coming fast.
First-in-Class Drugs: Breaking New Ground
Almost half of the 2024 approvals-24 out of 50-were first-in-class. That means they work in ways no other drug has before. They’re not copies. They’re new tools built from fresh science.
Take Cobenfy (xanomeline and trospium chloride). Approved in September 2024, it’s the first new schizophrenia treatment in 27 years. Older drugs target dopamine. Cobenfy targets muscarinic receptors instead. In trials, it improved symptoms by 34% compared to placebo. Side effects? Nausea in 12%, constipation in 8%. That’s far lower than the 25% nausea and 18% constipation common with older antipsychotics. But it’s not risk-free. The FDA requires patient education on anticholinergic effects like dry mouth and blurred vision.
Then there’s Yorvipath (palopegteriparatide), approved in October 2024 for hypoparathyroidism. For decades, patients had to take calcium and vitamin D supplements daily. Many still had low calcium levels. Yorvipath mimics the body’s natural parathyroid hormone. At 24 weeks, 89% of patients hit target calcium levels without supplements. Side effects? Nausea (22%) and dizziness (15%). Much better than the 38% and 29% seen with old treatments.
Alzheimer’s Drugs: Progress with Caveats
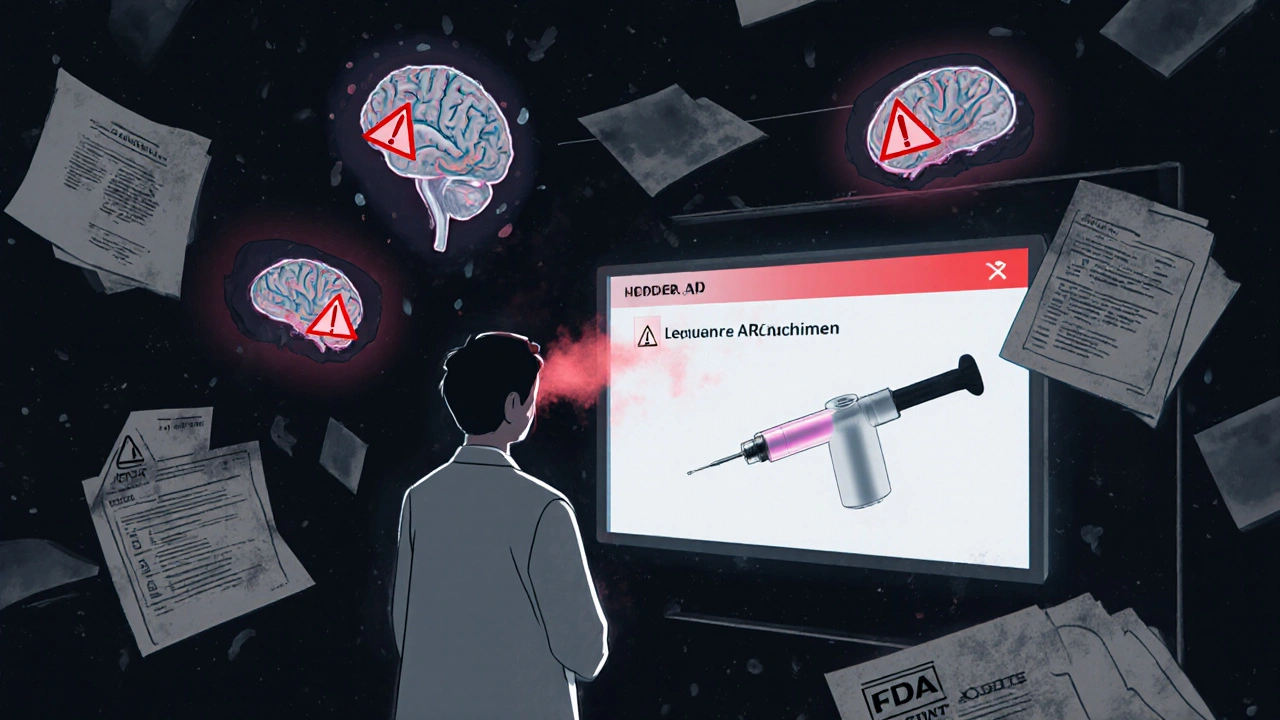
Two drugs now target amyloid plaques in Alzheimer’s: lecanemab (Leqembi) and donanemab-azbt (Kisunla). Both showed 30-35% slower cognitive decline over 18 months. That’s meaningful. But they come with serious risks.
Kisunla caused amyloid-related imaging abnormalities (ARIA) in 24% of patients in trials. That’s brain swelling or bleeding. In real-world use, early data from the FDA’s adverse event system shows ARIA rates are 5-7 percentage points higher than in trials-especially in people with two copies of the APOE ε4 gene. The FDA now requires strict monitoring protocols before and during treatment. If you’re considering Kisunla, you need an MRI before starting and regular follow-ups.
The good news? A subcutaneous version of Leqembi is expected in August 2025. It’s an injection you can give yourself at home. Same effectiveness. But injection site reactions (redness, swelling) are 31% more common. No increase in ARIA, though. That’s a big step toward convenience.

Emergency Medications: Simpler, Safer, Faster
Some of the most impactful approvals aren’t for chronic diseases. They’re for emergencies.
Neffy (epinephrine nasal spray), approved November 2024, gives people with severe allergies a needle-free option. In tests, 98% of untrained users got the dose right-compared to 87% with auto-injectors. That’s huge. But absorption is 15% slower. If you’re having a full-blown anaphylactic reaction, every second counts. Neffy works best for mild-to-moderate cases or if you’re not comfortable with needles. Real-world data shows a 22% higher chance of treatment failure in severe cases. So, it’s not a replacement for epinephrine auto-injectors in high-risk patients.
Zurnai (nalmefene nasal spray), approved December 2024, is the first nasal opioid overdose reversal drug with a longer half-life than naloxone. It lasts 6.2 hours versus 2.1. That means fewer repeat doses and fewer breathing complications. For people at risk of overdose, this could be life-saving. It’s already being stocked in harm reduction centers.
Repurposed Drugs: Old Medicine, New Uses
Sometimes, the best new drug is an old one used in a new way.
Zepbound (tirzepatide) was approved for weight loss. Now, it’s also approved for obstructive sleep apnea. In the SURMOUNT-OSA trial, patients saw a 46% drop in breathing interruptions per night. Weight loss was around 4.9%. Side effects? Gastrointestinal issues in 32%-mostly nausea and diarrhea. Nothing new. Just more of what we already knew from GLP-1 drugs.
Dupixent (dupilumab), originally for eczema and asthma, got approved for COPD in November 2024. It reduced moderate-to-severe flare-ups by 29%. But 17% of users had injection site reactions. Nine percent developed eosinophilia-higher than placebo. That’s not dangerous for everyone, but it needs watching. This shows how one drug can help multiple conditions, but safety must be re-evaluated each time.
Upcoming Approvals: What’s Coming in 2025
The pipeline doesn’t stop. Dozens of new drugs are waiting for FDA decisions.
Cardamyst (etripamil), a nasal spray for sudden rapid heartbeats (PSVT), could be approved by December 2025. In trials, 74% of patients returned to normal rhythm within 30 minutes. Side effects? Mostly nasal discomfort. No heart rhythm problems. Imagine being able to treat a scary heart episode at home-no ER visit needed.
Elinzanetant, for menopause hot flashes, has a PDUFA date of October 26, 2025. It cut hot flashes by 52% in trials. No blood clots. No breast cancer risk. Just headaches (18%), dry mouth (15%), and constipation (12%). This could be a safer alternative to hormone therapy for many women.
Wegovy (semaglutide) is getting an oral version. It’s not just for weight loss anymore. Trials show it reduces body weight by nearly 15% and lowers cardiovascular risk. The oral form works just as well as the injection. Side effects? Still mostly nausea and diarrhea-but now you don’t need needles. Approval expected late 2025.
How Safety Is Being Monitored After Approval
Approval isn’t the end. It’s the beginning of real-world monitoring.
The FDA now requires 24% of new drugs to have mandatory post-approval safety studies. That’s up from 17% in 2023. These studies track long-term effects in diverse populations-something clinical trials often miss.
For Kisunla, the FDA issued a safety communication in June 2025 after real-world data showed higher ARIA rates. For Neffy, early reports show more treatment failures in severe reactions. These aren’t failures of the system. They’re proof it’s working.
Drugmakers must now report adverse events faster. The FDA is also working with the European Medicines Agency to share safety data globally. That means we’ll know sooner if a drug causes unexpected problems in other countries.
What This Means for Patients and Doctors
These new drugs are powerful. But they’re not magic bullets.
Doctors are asking for more training. A Sermo survey found 68% of clinicians requested extra education on at least one 2024-approved drug. Why? Because the mechanisms are complex. Cobenfy isn’t just another antipsychotic. Yorvipath isn’t just another calcium pill. You can’t treat them the same way.
Patients need to ask: What’s the real benefit? What are the risks? Do I need special monitoring? If you’re prescribed a new drug, ask your doctor about the REMS program. Some drugs-like Kisunla-require you to enroll in a safety program. That’s not bureaucracy. It’s protection.
The American Medical Association now recommends documenting shared decisions. If you’re starting a new drug, make sure your doctor writes down why they chose it-and what alternatives were considered. That’s your right.
Final Thoughts: Innovation with Responsibility
The pace of drug development is accelerating. The science is exciting. But safety can’t be an afterthought. The best drugs aren’t just the ones that work. They’re the ones that work safely-and where the risks are understood, managed, and communicated.
2024 and 2025 are turning points. We’re not just getting more drugs. We’re getting smarter ones. And the system is getting better at watching over them. That’s progress.





Nicole Ziegler
November 21, 2025 at 02:59
Cobenfy sounds like a game-changer 😍 I’ve got a cousin with schizophrenia and the side effects of the old meds were brutal. This feels like hope with fewer side effects. Still, gotta watch for dry mouth-my grandma’s been on anticholinergics and she’s basically a desert.