Eflornithine Production: How It's Made and Why It Matters
When you hear eflornithine, a specialized medication used to slow unwanted facial hair and treat African sleeping sickness. Also known as DFMO, it's not a drug you find on every pharmacy shelf—because making it is hard, expensive, and tightly controlled. Unlike common generics, eflornithine isn’t mass-produced. It’s made in small batches by a handful of facilities worldwide, mostly in Europe and Asia, using multi-step chemical synthesis that requires precise temperature control, pure reagents, and strict contamination checks.
The core of eflornithine production, the process of synthesizing the active pharmaceutical ingredient (API) from precursor chemicals. Also known as DFMO synthesis, it involves reacting ornithine derivatives with specific halogenated compounds under controlled conditions—a process that’s sensitive to impurities. Even tiny errors can create toxic byproducts, which is why the FDA and EMA audit these facilities closely. You won’t find this kind of manufacturing in every Chinese API plant, even though China produces 80% of the world’s generic drug ingredients. Eflornithine is different. It’s a narrow therapeutic index drug, meaning the difference between a safe dose and a harmful one is small. That’s why only certified labs with validated processes are allowed to make it.
What makes eflornithine production even trickier is that demand is low but critical. It’s used for a rare skin condition called hirsutism and a deadly parasitic disease called trypanosomiasis. Because of this, production runs are infrequent, and supply chains are fragile. One factory shutdown, a raw material shortage, or a regulatory delay can leave patients without treatment. That’s why you’ll see articles on this site about medication shortages, generic drug quality, and API manufacturing risks—they’re all connected. When a drug like eflornithine is hard to make, it’s easy to lose.
There’s no shortcut here. Unlike aspirin or metformin, you can’t just copy the formula and scale up. The synthesis requires specialized equipment, trained chemists, and years of process validation. Even authorized generics of eflornithine must meet the same exacting standards as the original brand. That’s why when a batch fails purity tests—like the nitrosamine contamination issues seen in other generics—it’s pulled immediately. Patient safety isn’t just a policy; it’s baked into every step of the process.
So if you’re wondering why eflornithine is so hard to get, or why prices jump when supplies run low, it’s not just about profit. It’s about science, regulation, and the fragile balance between making a life-saving drug and doing it right. Below, you’ll find real-world posts that dig into the broader issues behind drugs like this—how manufacturing flaws happen, how regulators respond, and what patients can do when the supply vanishes.
The Environmental Impact of Eflornithine Production
Eflornithine saves lives from sleeping sickness, but its chemical production generates toxic waste and high emissions. A greener method exists - but isn't being used. Here's why.
About
Medications
Latest Posts


Medicaid and Generics: How Low-Income Patients Save Hundreds on Prescription Drugs
By Orion Kingsworth Feb 18, 2026
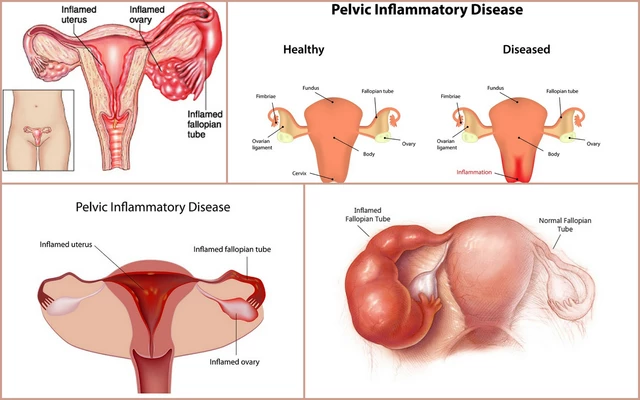
The use of clavulanate in the management of pelvic inflammatory disease
By Orion Kingsworth May 27, 2023

Nitrosamine Contamination in Generic Drugs: Recent Recalls and Regulatory Shifts
By Orion Kingsworth Nov 17, 2025

