New Drug Approvals: What’s Coming, Why It Matters, and How It Affects You
When you hear new drug approvals, the official green light from regulators like the FDA for medications to enter the U.S. market. Also known as drug clearance, it’s not just paperwork—it’s the moment a new treatment becomes available to real people dealing with real health problems. These approvals don’t happen in a vacuum. They’re tied to everything from generic drugs, affordable copies of brand-name medications that make treatment accessible to drug safety, the ongoing monitoring of side effects, contamination risks, and how well a drug actually works outside clinical trials. And when a new drug hits the market, it doesn’t just change prescriptions—it changes lives.
Take nitrosamine contamination, for example. Since 2018, over 500 FDA recalls have been tied to this hidden chemical in generic drugs. That’s not a glitch—it’s a system failure. When new approvals happen without enough scrutiny of manufacturing quality, especially from global suppliers like China, which makes 80% of the world’s drug ingredients, patient safety is at risk. The same goes for authorized generics: they’re chemically identical to brand-name drugs but sold under a different label. They’re not cheaper just because they’re generic—they’re cheaper because the brand company itself makes them. That’s a trick most people don’t know about. And when a generic fails to work as expected—like with narrow therapeutic index drugs such as warfarin or levothyroxine—it’s not a coincidence. It’s a gap in bioequivalence testing that new approvals often ignore.
Meanwhile, the FDA’s safety alerts for generics often lag behind brand-name drugs. Why? Because labeling rules haven’t kept up with how drugs are actually used. A patient on a generic version of a drug might never see the latest warning because the label hasn’t been updated. That’s why tracking new drug approvals isn’t just for doctors or pharmacists—it’s for anyone taking medication. Every approval comes with a story: the rare but deadly aplastic anemia linked to chloramphenicol, the opioid-induced itching that antihistamines can’t fix, or the environmental cost of producing a lifesaving drug like eflornithine. These aren’t abstract issues. They’re the hidden consequences behind every pill you swallow.
What you’ll find below isn’t just a list of articles. It’s a map of what’s really happening in drug development, regulation, and patient safety. From how states push generic use to cut costs, to why some generics don’t work, to how AI tools like OpenFDA let you dig into side effect reports yourself—this collection shows you how to read between the lines of pharmaceutical news. You don’t need a medical degree to understand what’s safe, what’s risky, and what’s being hidden. You just need the right information.
New Drug Approvals 2024-2025: What’s Approved and How Safe Are They?
Explore the latest FDA-approved drugs from 2024-2025, including Alzheimer's treatments, emergency medications, and breakthrough therapies. Learn how they work and what their real-world safety profiles reveal.
About
Medications
Latest Posts
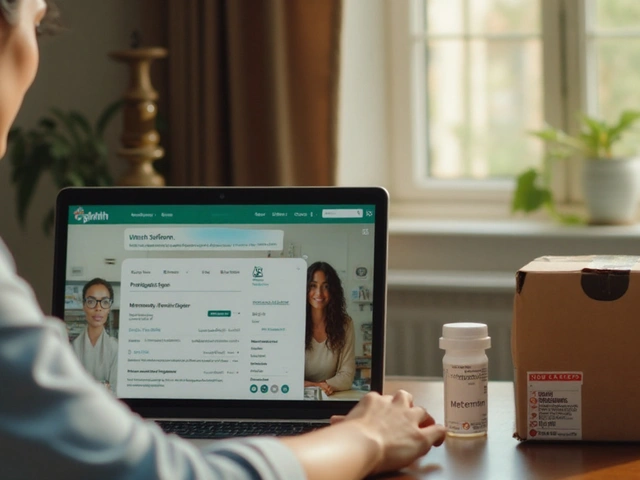
How and Where to Buy Metformin Online Safely (2025 Guide)
By Orion Kingsworth Aug 23, 2025
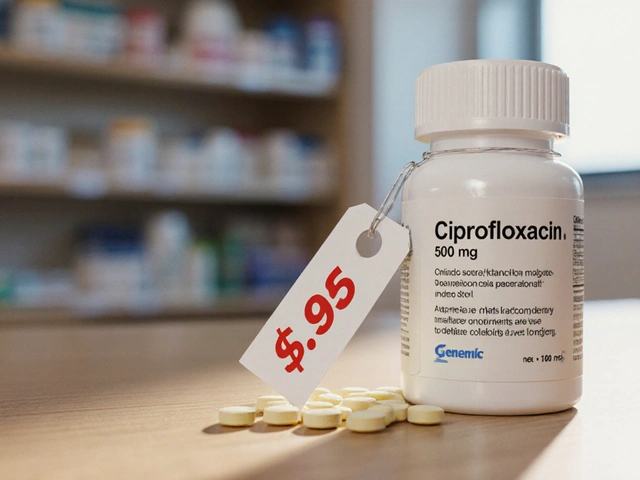
Buy Cheap Generic Ciprofloxacin Online - Safe 2025 Guide
By Orion Kingsworth Oct 7, 2025

Compare Isofair (Isotretinoin) with Alternatives for Acne Treatment
By Orion Kingsworth Nov 1, 2025

