Authorized Generics: What They Are, Why They Matter, and When They Don’t Work
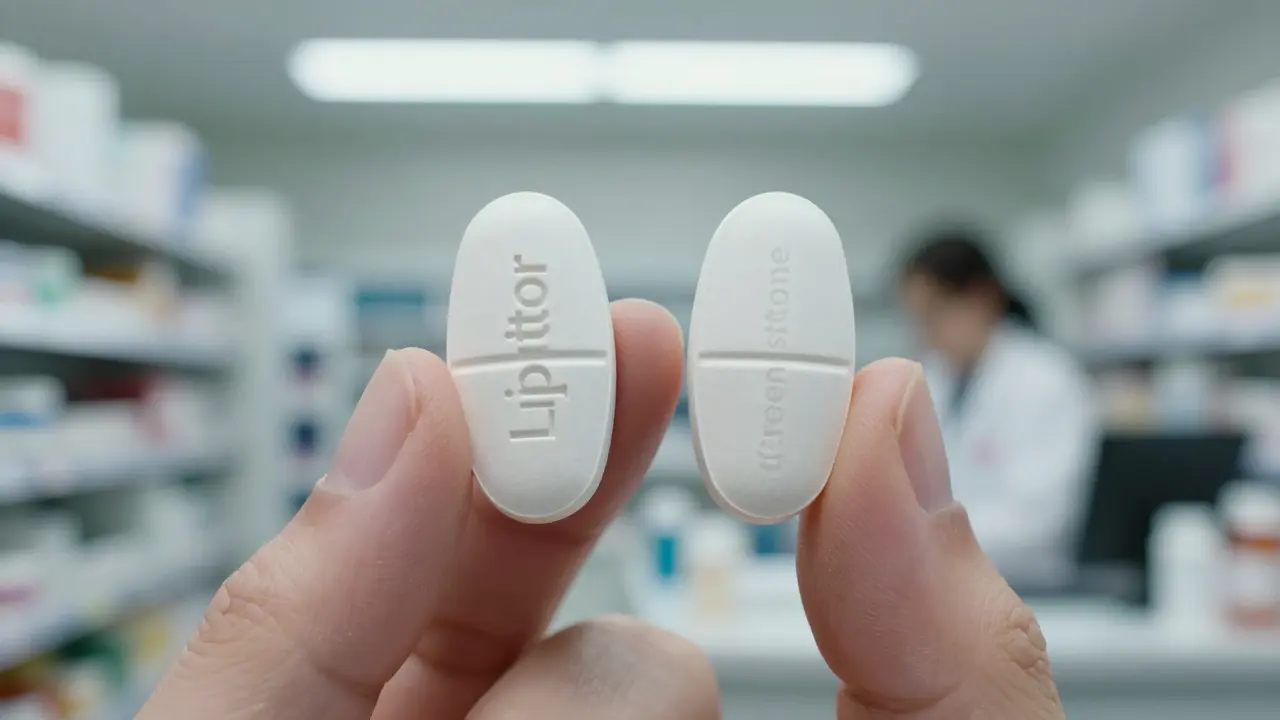
When you hear authorized generics, brand-name drugs sold under a generic label, made by the original manufacturer under the same FDA-approved process. Also known as brand-name generics, they’re not knockoffs—they’re the real thing, just cheaper. Unlike regular generics, which are made by different companies using the same formula, authorized generics come from the same factory, same equipment, same batch records as the brand-name version. That means the active ingredient, the fillers, even the coating on the pill—everything—is identical. No guesswork. No trade-offs.
But here’s what most people don’t know: generic drugs, medications approved by the FDA as bioequivalent to brand-name versions can still vary in how they behave in your body—especially if they’re not authorized. A 2020 study found that for drugs with a narrow therapeutic index—like warfarin or levothyroxine—switching between different generic manufacturers sometimes caused measurable changes in blood levels. That’s why authorized generics exist: they remove the uncertainty. If your doctor prescribes Lipitor, and you get an authorized generic made by Pfizer, you’re getting the exact same pill. No surprises.
Still, drug pricing, the cost of medications influenced by manufacturing, patents, and market competition doesn’t always reflect quality. Some authorized generics cost nearly as much as the brand name because the original company controls the market. Others drop to pennies on the dollar once competition kicks in. And while authorized generics avoid the quality issues seen in some overseas-made generics—like the nitrosamine contamination linked to over 500 recalls since 2018—they’re not always available. If your pharmacy doesn’t stock them, you might get a regular generic instead. That’s not bad, but it’s not the same.
And then there’s the problem of bioequivalence, the standard that proves a generic drug performs the same way in the body as the brand version. The FDA says generics must be within 80–125% of the brand’s absorption rate. Sounds tight, right? But for some people, even a 5% difference can mean a seizure, a blood pressure spike, or a flare-up of chronic pain. That’s why some patients swear their generic doesn’t work—sometimes, it’s not the drug. It’s the filler. The coating. The way it dissolves. Authorized generics sidestep that because they’re built the same way as the brand. No hidden variables.
You won’t find authorized generics on every prescription. They’re not always offered. And if you’re on Medicaid or Medicare, your plan might not even list them. But if you’ve ever switched to a generic and felt something off—drowsier, less effective, more side effects—you should ask. Not all generics are created equal. And if your doctor agrees, an authorized generic might be the quiet fix you’ve been missing.
Below, you’ll find real stories and data about when generics fail, why some drugs are safer as authorized versions, how manufacturers cut corners, and what regulators are—or aren’t—doing about it. No fluff. Just what you need to know to make sure your medication works the way it should.
Authorized Generics: Same Drug, Different Label - What You Need to Know
Authorized generics are the exact same drug as brand-name medications, just sold under a different label. Learn how they work, why they exist, and how they can save you money without sacrificing quality.
What Are Authorized Generics? Complete Explanation
Authorized generics are identical to brand-name drugs but sold without the brand label. Learn how they work, how they differ from regular generics, and why pharmaceutical companies use them to stay competitive.
About
Medications
Latest Posts


Buy Generic Lipitor (Atorvastatin) Online Cheap in 2025: Safe Options, Prices, and Tips
By Orion Kingsworth Sep 9, 2025
The science behind reemerging influenza: understanding virus mutations
By Orion Kingsworth May 15, 2023

Amoeba Infections 2025: Clinical Guide for Healthcare Professionals (Diagnosis, Treatment, Prevention)
By Orion Kingsworth Sep 4, 2025