Brand Name Drugs: What They Are, Why They Matter, and When Generics Fall Short
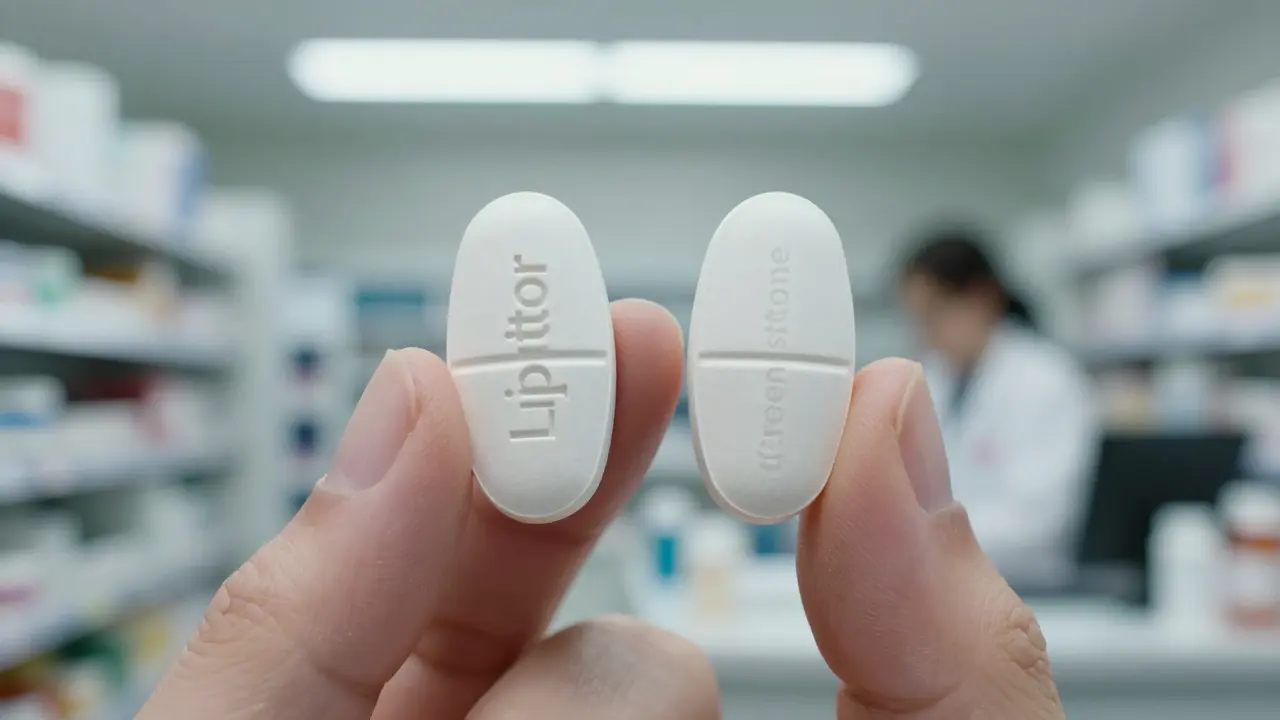
When you hear brand name drugs, the original medications developed and patented by pharmaceutical companies before generics enter the market. Also known as originator drugs, they’re the first version of a medicine that goes through full clinical testing and gets FDA approval based on proven safety and effectiveness. These aren’t just fancy labels—they’re the baseline for how a drug should work in your body. Think of them as the blueprint. Once the patent expires, other companies can make copies called generics. But copies aren’t always the same. Sometimes, even if a generic meets FDA standards, it doesn’t behave the same way in real people. That’s not a myth—it’s documented in cases where patients on epilepsy meds, thyroid drugs, or blood thinners suddenly had problems after switching to a generic.
The real issue isn’t that generics are bad. It’s that drug safety, the ongoing monitoring of how medications perform after they’re on the market doesn’t always catch subtle differences. The FDA tracks serious side effects and recalls, but it doesn’t routinely test whether a generic works just as well for every person. That’s why you’ll see posts here about therapeutic failure, when a medication stops working as expected, even though it’s chemically identical to the brand version. One person’s life-saving dose of levothyroxine becomes ineffective after a switch. Another’s seizure control slips after switching from brand-name Keppra to a generic. These aren’t rare outliers—they’re signals that the system has gaps.
And it’s not just about effectiveness. FDA approvals, the official process that clears a drug for sale in the U.S. apply differently to brand and generic drugs. Brand name drugs go through years of clinical trials before approval. Generics only need to prove they’re "bioequivalent"—meaning they release the same amount of active ingredient into the bloodstream within a certain window. That’s fine for most drugs. But for medicines with a narrow therapeutic index—where the difference between a helpful dose and a toxic one is tiny—those small variations matter. That’s why nitrosamine contamination, inconsistent manufacturing in China, or even changes in inactive ingredients can trigger recalls or unexpected reactions.
You’ll find posts here that dig into why some people still need the brand version, how states push generics to cut costs—and sometimes, how that backfires. You’ll see how drug labeling lags for generics, why some patients get stuck with pills that don’t work, and what to do if your medication suddenly stops helping. This isn’t about picking sides. It’s about knowing the difference between what’s legally approved and what actually works for you. The truth is, your body doesn’t care about patents. It cares about results. And if your health depends on a drug working exactly right, you deserve to know when the brand name might still be the better choice.
Authorized Generics: Same Drug, Different Label - What You Need to Know
Authorized generics are the exact same drug as brand-name medications, just sold under a different label. Learn how they work, why they exist, and how they can save you money without sacrificing quality.
Drug Interactions: Same Risk for Generic and Brand Medications
Generic and brand-name drugs have the same active ingredients, so their risk of drug interactions is essentially identical. Learn what really matters - and what myths to ignore - when choosing between generics and brands.
What Are Authorized Generics? Complete Explanation
Authorized generics are identical to brand-name drugs but sold without the brand label. Learn how they work, how they differ from regular generics, and why pharmaceutical companies use them to stay competitive.
About
Medications
Latest Posts


NSAID Sensitivity and Asthma: What Patients Should Watch
By Orion Kingsworth Nov 28, 2025

Rasagiline’s Role in Slowing Parkinson’s Disease Progression
By Orion Kingsworth Oct 21, 2025

Dyskinesias and Biofeedback: A Promising Treatment Option
By Orion Kingsworth Mar 16, 2025